Venture into the fascinating realm of heat treatment of metals. Through this guide, become informed about the transformative methods used to enhance metal characteristics. Discover, understand, and appreciate the expertise in this vital industrial process.
Principles of Heat Treatment!
Basic Heat Treatment Theory

Heat treatment of metals involves three steps: heating, soaking, and cooling. Metals reach certain temperatures (800-1400°F), then dwell at that heat level for a period.
After that, cooling happens. Sometimes, rapid cooling, called quenching, changes the metal’s structure, enhancing hardness. Meanwhile, slow cooling creates softer, more flexible metals.
Heat treatment aims to change the metal’s properties for better usability in different industrial applications.
Relevance of Thermodynamics in Heat Treatment
Thermodynamics plays a vital role in heat treatment. Metals absorb heat, causing atoms to move and rearrange themselves, transforming the metal structure. So, a shift in heat alters the energy state of the metal, leading to different properties.
Heat treatment revolves around the control of these energy states. Understanding thermodynamics, especially enthalpy (H), entropy (S), and Gibbs free energy (G), is crucial for predicting changes and optimizing heat treatment processes.
Heat Treatment Processes!
Overview of Various Heat Treatment Processes
§ Annealing
Annealing is a prime process to define heat treatment of metal. Metals, heated to certain temperatures, cool down slowly. Microstructures soften, enabling enhanced workability. ASTM A681-08 and SAE J406 are prime annealing standards.
Understanding them helps control hardness, toughness, and elasticity. Annealing employs industrial furnaces for the heat treatment of metals, which ensures precise temperature control.
§ Normalizing
Normalizing follows annealing closely. Heating metals to specific temperatures, then allowing air cooling creates uniform microstructures. This process helps adjust mechanical properties. Standards like ASTM A255-10 and ISO 683-1:2016 guide normalizing. Expect increased strength and hardness but less ductility after normalizing.
§ Hardening
Hardening, a crucial part of heat treatment of metals, transforms metals to their hardest state. Metals heated to set temperatures, quenched quickly in liquids, achieve this. Standards like ISO 4957:2018 guide hardening processes. Expect the hardest, yet brittle, metals after hardening.
§ Quenching
Quenching hails as a rapid cooling process post hardening. This step involves immersing hot metals in a liquid or gas quickly. It leads to increased hardness.
ASM International and AISI have guidelines for quenching. Quenching minimizes brittleness, but risk of cracking exists if not tempered correctly.
§ Tempering
Tempering follows quenching closely. Reheating the quenched metals to lower temperatures, then cooling, achieves this. Metals gain improved ductility, reducing brittleness. ASTM A255-10 and ISO 4957:2018 guide tempering. The outcome balances hardness and ductility, for metals that can withstand stress and strain.
§ Case Hardening
First, expose the metal surface to carbon atoms. Then, heat to 900°C. And finally, rapidly cool. It’s also known as quenching. This gives a hard shell outside, with a tough core inside. Essential for gears, it improves wear resistance while maintaining toughness.
§ Carburizing
It is similar to case hardening, but with more carbon. The part, placed in a carbon-rich atmosphere, heats to between 850 to 950°C. After soaking, the part undergoes quenching. Results show improved hardness and increased life span of metal parts.
§ Nitriding
Nitrogen atoms play a significant role here. Heat the metal to 500 to 600°C in a nitrogen-rich atmosphere. By letting nitrogen atoms penetrate the metal surface, you increase hardness and fatigue strength. It’s an effective method for heat treatment of metal 3D printing parts.
§ Cyaniding
It’s a quick, efficient method. Introduce the metal to a salt bath, at 800 to 900°C. The bath contains sodium cyanide. After soaking, rinse and quench. Benefits include a hard surface and a short heat time.
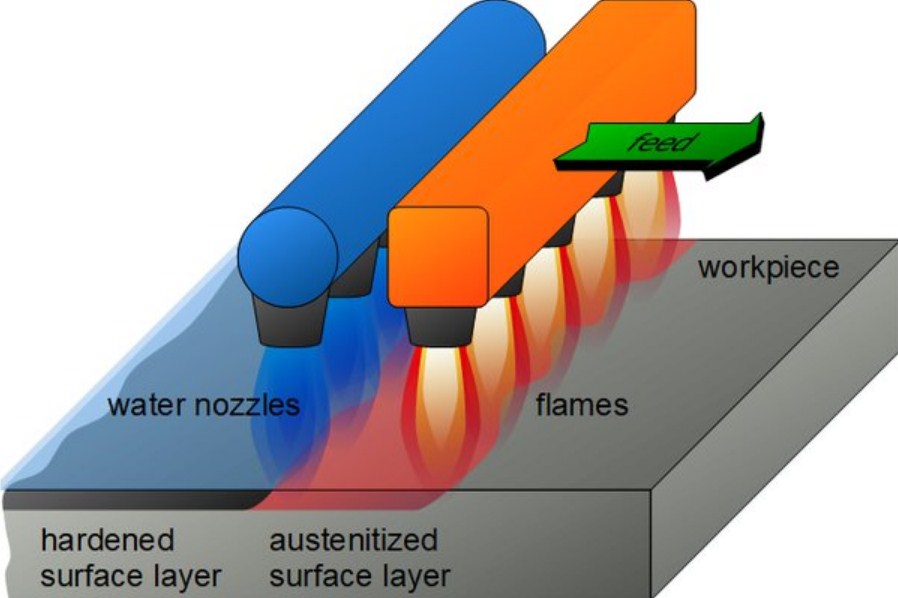
§ Flame Hardening
Utilize a high-heat flame for this. Rapid heating and quenching only affect the metal’s surface. Leaving the core relatively cool gives a tough interior and a hard exterior. Flame hardening provides control over the hardened area. Good for large components, like gears or shafts.
§ Induction Hardening
Induction Hardening forms part of heat treatment of metals and alloys. Under a high-frequency magnetic field, heat is produced inside the metal. In seconds, the surface hardens. By rapidly cooling, the core stays soft. Notably, the method improves hardness, wear resistance, and fatigue life.
§ Spheroidizing
Spheroidizing, a heat treatment of metal, is key in making the metal softer. Heated below critical temperature for a longer period, carbides transform into globules. The globular structure fosters machine-ability in high-carbon steels.
§ Martempering
In Martempering, the metal gets quenched from austenitizing temperature into a bath. The bath keeps the metal till it cools uniformly. Later, the metal is air-cooled. This lessens internal stresses, distortion, and cracking. Metals become tougher and more ductile, showing what is the effect of heat treatment on a metal.
§ Austempering
Austempering, a type of metal heat treatment, improves strength and toughness. Here, the metal is quenched from a high temperature in a bath. Then, it’s held till transformation occurs. Austempering results in a structure called bainite, leading to superior toughness and strength.
§ Stress Relieving
Stress Relieving is critical to remove internal residual stresses from metals. Metals undergo heating at lower temperatures and then cooling. This process reduces the risk of dimensional changes during further manufacturing steps.
§ Cold Working
Not exactly a heat treatment of metals, but crucial in metalworking. Deforming metals below recrystallization temperature enhances strength. However, metal becomes more brittle. The process results in work hardening, indicating the versatility of different types of heat treatment of metals.
Heating Methods in Heat Treatment
§ Conduction Heating
In conduction heating, metal touches a hot object. Metal atoms get excited, then pass the heat to their neighbors. Noteworthy conduction heating: steel molds at 900°F. Remarkably, no fan or flame needed.
That’s the simplicity of conduction heating. Yes, precision is required. Temperature control is vital. Too much heat, metal distorts. So, pay attention to details.
§ Convection Heating
It’s time to understand conduction’s cousin – convection. It involves gases or liquids. Picture a furnace. Hot air rises, warms the metal. The air then cools down, sinks, and gets reheated. Round and round it goes. It’s the principle behind industrial kilns.
Yes, these large ovens reach 2000°F. They alter metal properties, crafting durable parts.
§ Radiation Heating
The sun’s heat reaches us through radiation. Apply that idea to metal treatment. Emit infrared rays onto a metal. Heat gets absorbed, raises the temperature.
Perfect for large, flat surfaces. The method is efficient, direct, and fast. In industry, massive panels undergo radiation heating. Soldering, for example, commonly employs this method.
§ Induction Heating
This method uses electromagnetism. Wrap a coil around the metal. Send alternating current through the coil. This excites the metal atoms, creates heat. Copper, aluminum, steel, all are suitable candidates. Crucially, the process is quick, clean, controlled.
§ Direct Resistance Heating
Connect the metal to an electricity source. The resistance to the electric current generates heat. This is especially effective for high-resistance metals, such as tungsten and stainless steel fabrication. There’s no heat loss during transmission, making the process energy-efficient. It’s widely used in the manufacturing industry, due to the benefits of heat treatment in metals.
§ Infrared Heating
The use of Infrared Heating is an integral part of the heat treatment of metals. In this process, radiant heat waves directly penetrate the metal. The surface absorbs this heat, enhancing properties like hardness and toughness.
This method offers rapid, precise, and uniform heating, proving efficient in the energy-intensive industry. Such treatment fits ideally in a process called case hardening heat treatment of metals.
§ Laser Heating
Laser heating harnesses concentrated beams of light to heat metals. Its focusability and high power make it suitable for processes like welding and annealing. This method minimizes deformation, ensuring optimal part shapes, a vital factor in checks after heat treatment of metals.
§ Electron Beam Heating
The Electron Beam Heating technique relies on a stream of high-speed electrons striking the metal. The kinetic energy transforms into heat, offering deep penetration and precise control.
§ Vacuum Heating
Vacuum Heating in the heat treatment of metals involves exposing materials to high temperatures in vacuum conditions. This technique curbs oxidation and contamination.
Given the cost of metal heat treatment, this technique offers a cost-effective alternative. Its usage is widespread in industries requiring cryogenic heat treatment of metals.
| Heating Method | Heat Source | Efficiency (%) | Heat Transfer Medium | Max Temperature (°C) | Application | Limitations |
| Conduction Heating | Direct contact | 70-90 | Solid objects | 1200 | Metals, glass | Requires physical contact |
| Convection Heating | Air, fluid | 50-70 | Gases, liquids | 350 | HVAC, ovens | Inefficient in vacuum |
| Radiation Heating | Electromagnetic waves | 80-95 | Vacuum, air | 3000 | Solar panels, heaters | Distance-dependent |
| Induction Heating | Electromagnetic field | 85-95 | Magnetic material | 3000 | Metal hardening, welding | Only for magnetic materials |
| Direct Resistance Heating | Electric current | 80-95 | Conductive materials | 2800 | Metal forming, glass manufacture | High energy costs |
| Infrared Heating | Infrared radiation | 85-90 | All types of materials | 3000 | Curing paints, drying | Requires line of sight |
| Microwave Heating | Microwave radiation | 60-75 | Dielectric materials | 300 | Cooking, sterilization | Not effective for metals |
| Laser Heating | Laser light | 90-95 | All types of materials | 6000 | Cutting, drilling metals | High initial costs |
| Electron Beam Heating | Electron beam | 90-95 | Vacuum, gases | 30000 | Welding, surface modification | Requires vacuum |
| Vacuum Heating | Absence of air | 60-80 | Metals, ceramics | 3500 | Heat treatment of alloys | Slow heat transfer |
Table on Heating Methods in Heat Treatment
Metal Properties and Heat Treatment!

Effects of Heat Treatment on Mechanical Properties of Metals
·Hardness Enhancement
Heat treatment of metals often targets hardness enhancement. By heating a metal like steel to 900°C, and then cooling it rapidly, the process called “quenching” is enacted.
As a result, steel attains a hardened state, suitable for cutting tools. The hardness of the metal escalates, making it more resistant to deformation and wear.
·Strength Increase
The strength of metals also sees considerable improvement with heat treatment. Through a process known as “tempering”, metals such as iron are heated to temperatures between 200°C and 500°C. Slow cooling afterwards increases the overall strength of the metal. Such metals find use in robust structures and machines.
·Ductility Reduction
Heat treatment can also reduce a metal’s ductility, or its ability to stretch without breaking. Aluminum, for instance, becomes less ductile after prolonged exposure to temperatures above 400°C. Although reduced ductility might sound negative, it sometimes enhances a material’s usability in specific applications.
·Toughness Improvement
Toughness, or a material’s ability to absorb energy before fracturing, can also be improved through heat treatment. By repeatedly heating and cooling a metal like copper, it’s possible to increase its toughness significantly. The effects of repeated heat treatment on metals include better performance under high-stress situations.
·Elasticity Modification
Heat treatment can modify a metal’s elasticity, or its ability to return to its original shape after deformation. By varying the heat treatment temperature, the elasticity of metals such as titanium can be modified to fit specific application requirements. An example is aircraft construction, where high elasticity is often desired.
·Plasticity Change
The definition of the heat treatment of metal involves altering metals under heat, affecting their plasticity. High temperatures allow atoms in a metal to become more mobile, changing the metal’s shape without breaking it.
For example, a blacksmith heats iron to make it malleable. After cooling, the iron maintains its new form, demonstrating altered plasticity.
·Fatigue Resistance
Heat treatment can improve a metal’s resistance to fatigue. For example, a metal part in an automobile engine can withstand repeated cycles of stress without breaking due to heat treatment. Such a process mitigates fatigue cracks that would otherwise develop, thereby enhancing the durability of the component.
·Fracture Toughness
The effect of heat treatment on properties of metals like fracture toughness is noteworthy. Post-treatment, metals can withstand high impact forces without fracturing. This makes them ideal for use in high-stress environments like airplane wings or bridge structures where resistance to cracking under strain is vital.
·Wear Resistance
Heat treatment also plays a critical role in enhancing wear resistance of metals. Metals exposed to heat treatment have their surface hardness increased. This process renders the metal capable of withstanding prolonged periods of friction and wear.
·Impact Resistance
A key factor in the different types of metal heat treatment is their influence on impact resistance. Heat treatment modifies the microstructure of the metal, thus improving its ability to absorb energy during impacts.
Alteration of Physical Properties Through Heat Treatment
·Color Change
One must understand that heat treatment of metals can cause a color shift. For instance, steel turns blue at around 300°C. This process is known as tempering. Interestingly, gun metal can attain a rainbow hue, a process known as gun metal heat treatment coloring name of process.
·Magnetic Property Alteration
In the heat treatment process of metals, magnetic properties may be altered. Steel, at 770°C, loses its magnetism – a phase called the Curie point. Heat treatments control the magnetic behavior of metals.
·Electrical Conductivity Change
Heat treatment methods of metals significantly impact electrical conductivity. As an example, copper’s electrical conductivity improves with annealing, a type of heat treatment, as it increases lattice vibrations, resulting in a higher conductivity.
·Thermal Conductivity Modification
Thermal conductivity in metals also changes with heat treatments. Aluminum, for instance, shows increased thermal conductivity post-heat treatment due to crystal structure stabilization, which enhances heat flow.
·Density Variation
The heat treatment process influences a metal’s density as well. Metals, when subjected to heat treatment, might exhibit density changes due to atomic arrangement modifications, impacting the overall mass per unit volume.
·Crystalline Structure Alteration
Crystalline structure is another property that heat treatment alters. In fact, heat treatment of ferrous and nonferrous metals leads to changes in the arrangement of atoms, resulting in different crystal structures. This, in turn, impacts a metal’s mechanical properties.
·Dimensional Stability
Stability is critical in heat treatment of metal AM parts. Proper heat treatment boosts stability. Certain metals, like Invar 36, have low expansion rates at high temperatures.
Invar 36 remains stable during heat treatments of up to 260°C. Technicians must maintain tight control of the process to ensure stability.
·Surface Appearance
Heat treatment affects the surface of metals. During heat treatment of metals annealing, the surface may darken due to oxidation. For instance, stainless steel sheet turns blue or brown. However, further polishing can restore the original appearance.
·Reflectivity Modification
The reflectivity of metallic materials can change due to heat treatment. For instance, in heat treatment of metallic materials, aluminum’s reflectivity can improve when heated at 400°C.
·Specific Heat Capacity
The specific heat capacity of metals is vital during treatment. For instance, copper has a capacity of 0.385 Joules per gram per degree Celsius. Through heat treatment, the heat capacity can increase, thus, better performance under high temperatures.
·Lattice Parameter Alteration
In the realm of heat treatment of metals, lattice parameters often undergo changes. By heating metals to temperatures nearing melting points, atom spacing alters. In a cubic crystal structure, this action shifts the arrangement.
As temperature lowers, so do parameters. Lattice adjustments occur due to interplay between thermal vibrations and interatomic forces. A controlled process, it guarantees improved performance and durability.
·Coefficient of Thermal Expansion (CTE)
During heat treatment, metals experience an alteration in CTE. CTE determines how a material expands when subjected to temperature changes.
Different metals possess distinct CTE values. Metals like aluminum have high CTE, expanding significantly under heat. Steel, on the other hand, has a lower CTE. Controlled heat application during treatment adjusts CTE, optimizing product quality.
·Elastic Modulus Variation
Elastic modulus, a measure of material stiffness, can alter through heat treatment.
The process reduces this modulus, making the metal more flexible. Lowering the elastic modulus enhances the metal’s malleability. Such manipulation forms an essential part of metalworking industries, such as automotive and aerospace.
·Corrosion Resistance
One of the main goals of heat treatment is enhancing corrosion resistance. Metals like aluminum and stainless steel naturally resist corrosion. With the heat treatment process, other metals can also increase their corrosion resistance.
This property is essential for metals used in environments prone to humidity and water exposure, ensuring longer lifespan and enhanced performance.
Enhancement of Chemical Properties via Heat Treatment
·Chemical Stability
In the realm of heat treatment of metals definition, chemical stability stands tall. Altering the heating cycle alters the metal’s reactivity, henceforth boosting stability.
In complex processes, such as carburizing or nitriding, extra elements blend with the metal, ensuring improved resistance to degradation.
·Oxidation Resistance
The most beneficial aspect of the heat treatment of metals experiment lies in refining oxidation resistance. Metals, when heated, develop a thin, protective oxide layer. This layer guards the metal against potential environmental oxidation, subsequently extending its lifespan significantly.
·Hydrogen Embrittlement Prevention
The process of metal heat treatment also works effectively against hydrogen embrittlement. By applying specific heat treatments, metals can release trapped hydrogen. The result is metals become less brittle and more ductile, boosting their overall performance.
·Sulfide Stress Cracking Resistance
Another vital feature of heat treatment involves enhancing resistance to sulfide stress cracking. Carefully controlled heat exposure minimizes the risk of crack formation due to sulfides, maintaining structural integrity, even under severe stress conditions.
·Acid Resistance
Acid resistance gets a significant boost with a correctly executed heat treatment of metals surface hardening process. By carefully managing heat levels, metals can develop a higher threshold against corrosive acid attacks, enhancing durability in chemically aggressive environments.
·Alkali Resistance
Alkali resistance is another aspect that can be optimized through heat treatment. By altering the metal’s microstructure via heat, it’s possible to enhance the metal’s resistance to alkali substances, expanding its applications in various industries.
·Chloride Resistance
A crucial feature in the heat treatment of metals bainite discussion is improved chloride resistance. Heated metals develop a protective layer, making them less vulnerable to corrosive chloride ions, extending their life and functionality in chloride-rich environments.
·Crevice Corrosion Resistance
In the heat treatment of metals hardening, significant resistance to crevice corrosion is achieved. Metals undergo a phase transformation, raising the corrosion threshold. A treated metal like stainless steel can resist corrosion for up to 70 years, ensuring long-term application in corrosive environments.
·Pitting Corrosion Resistance
The heat treatment process bolsters the pitting corrosion resistance in metals. Subjecting metals to specific high temperatures modifies their crystalline structure. The result is a superior pitting corrosion resistance, where even a tiny defect won’t compromise the metal’s integrity.
·Galvanic Corrosion Resistance
Through heat treatment, metals gain robust galvanic corrosion resistance. The heat induces alterations in the metal’s microstructure, thereby preventing the likelihood of galvanic corrosion. As a result, treated metals withstand the test of time when paired with different metals.
·Intergranular Corrosion Resistance
Heat treatment of metals helps fortify against intergranular corrosion. By inducing bainite structures during the heat treatment of metals bainite, the boundaries between grains in the metal become more resistant to corrosive attacks, promoting a metal’s lifespan in aggressive environments.
.
·Cavitation Corrosion Resistance
In the heat treatment of metals definition, enhancing cavitation corrosion resistance is a key objective. As metals get heated to certain temperatures, they become more impervious to cavitation corrosion, a common problem in hydraulic systems and propellers.
·High-Temperature Corrosion Resistance
Heat treatment amplifies a metal’s resilience to high-temperature corrosion. During the heat treatment of metals experiment, certain metals exhibit superior corrosion resistance at elevated temperatures. Such metals are excellent choices for high-temperature applications like turbine blades, furnaces, and combustion chambers.
| Criteria | Chemical Stability | Oxidation Resistance | Hydrogen Embrittlement Prevention | Sulfide Stress Cracking Resistance | Acid Resistance | Alkali Resistance | Crevice Corrosion Resistance |
| Temperature Range | 500-800°C | 600-850°C | 650-900°C | 600-850°C | 500-700°C | 500-700°C | 550-800°C |
| Time Duration | 1-3hrs | 2-4hrs | 2-3hrs | 2-4hrs | 1-3hrs | 1-3hrs | 2-3hrs |
| Atmosphere | Inert/Nitrogen | Air/Oxygen | Hydrogen-Free | Inert/Nitrogen | Acidic | Basic | Moist/Chloride-Rich |
| Alloy Types | All Alloys | Fe & Ni based | All Alloys | Fe & Ni based | Most Alloys | Most Alloys | Stainless Steel & Ni Alloys |
| Heating Method | Furnace | Furnace/Oven | Furnace | Furnace | Furnace | Furnace | Furnace/Oven |
| Cooling Method | Air/Water Quenching | Slow Cooling | Slow Cooling | Air/Water Quenching | Slow Cooling | Slow Cooling | Air/Water Quenching |
| Impact on Material | Enhances Stability | Reduces Oxidation | Prevents Embrittlement | Enhances Resistance | Increases Acid Resistance | Increases Alkali Resistance | Enhances Resistance |
Table on Enhancement of Chemical Properties via Heat Treatment!
Ideas on Process Control in Heat Treatment!
§ Temperature Control
Precise control of temperature marks a critical aspect of the heat treatment of metals process. High heat, often above 1700°F, is crucial to alter metal characteristics. Thermocouples and pyrometers measure temperature, ensuring optimal results.
Minor fluctuations can lead to significant quality issues. Thus, mastering this parameter bolsters the efficiency and product reliability of the entire process. Ensuring a stable temperature curve contributes to improved metal performance.
§ Atmosphere Control
The gas environment plays a pivotal role in metal treatment. Each procedure necessitates a specific atmosphere to avoid intergranular oxidation. Nitrogen, hydrogen, and endothermic gas are typically employed in these processes. Atmosphere control preserves metal surface quality and prevents corrosion. Therefore, maintaining precise atmospheric conditions is essential to obtaining high-quality, corrosion-resistant metals.
§ Cooling Rate Regulation
Following heating, the cooling process needs careful monitoring. Rapid cooling, or quenching, hardens steel, while slower cooling rates induce annealing effects.
To ensure a successful heat treatment of metals, electrically heated equipment often uses regulated cooling techniques. Regulating cooling rates influences metal properties and performance, achieving the desired hardness and toughness.
§ Quenching Media Management
Metals are cooled using a variety of quenching media such as water, oil, or air. Selection depends on metal properties and desired characteristics. For instance, oil quenching is used for high-speed steels. Each quenching medium influences the cooling rate and consequently, the final product’s properties. Mastery of quenching media is crucial for achieving the target properties, as highlighted in many heat treatments of metals notes.
§ Heat Distribution
In the heat treatment of metals, managing heat distribution is critical. Uneven heating can lead to intergranular damage. An electrically heated furnace ensures uniform distribution. The temperature must range between 900 to 1200°C.
§ Equipment Calibration
During the heat treatment of metals, equipment calibration plays a pivotal role. Furnaces and pyrometers need periodic checks. These checks guarantee accurate readings, vital for successful treatment. Without calibration, there could be drastic errors in the heat treatment process.
§ Furnace Pressure Control
Controlling pressure in the furnace is another crucial step. Changes in pressure can affect the heating and cooling rates. Maintaining steady pressure ensures consistent results in the heat treatment of metals process.
§ Process Sequencing
Correct sequence in the heat treatment process ensures efficiency. Cooling, heating, and soaking stages must follow a strict order. This sequence helps to avoid structural faults in metals.
§ Automation Integration
Control systems are now becoming crucial in heat treatment of metals. Programmable Logic Controllers (PLCs) are often used, capable of controlling complex operations.
With PLCs, parameters like temperature, heating rate, and cooling rate get precision control. These systems decrease manual labor and increase the production rate and quality, offering significant benefits.
§ Data Acquisition Systems (DAS)
In a DAS, various sensors collect information about heat treatment parameters. These sensors collect temperature, pressure, and time data. The DAS processes this data, providing critical insights for refining the heat treatment process. This information guides the decision-making process, resulting in better control of the heat treatment process and improved metal properties.
Material Considerations in Heat Treatment!

- Material Composition
- Carbon Content
- Alloy Elements
- Impurities Influence
- Material Structure
- Grain Size
- Phase Composition
- Microstructure Analysis
- Material Defects
- Material Thickness
- Material Hardness
- Surface Condition
- Material Shape
- Material Weight
- Material Dimension
- Material Ductility
Enhancing Heat Treatment Efficiency!
Process Optimization
Optimal types of heat treatment process for metals can lead to improved outcomes. Proper planning includes choosing the best heating rate, ideal temperature (around 500°C for softening, 900°C for hardening), and cooling rate. Fine-tuning these parameters promotes metal strength and longevity. A smooth flow from the heating furnace to the quenching bath reduces the risk of irregularities. Always aim for meticulous process management, crucial for robust metal properties.
Energy Saving
The energy use in heat treatment of metals matters significantly. High temperatures and long durations mean more energy. Adopting eco-friendly furnaces, capable of heating up to 950°C with minimal energy use, proves beneficial. Also, consider insulation materials that can reduce heat loss. Choose ones that retain heat effectively, maximizing your energy savings. Remember, lower energy consumption leads to decreased expenses, contributing positively to your bottom line.
Waste Reduction
Managing waste in types heat treatment of metal processes is critical. During quenching, use water efficiently. A flow rate of 10 L/min could result in less water usage.
Also, consider recycling heat-treated metal scraps. An estimated 1.3 billion tons of scrap gets discarded yearly. By reusing these, you can reduce waste, aiding the environment. Continual commitment to waste minimization is a smart strategy in any heat treatment process.
Automation Integration
Embracing automation in various types of heat treatment on metals can enhance efficiency. Introduce computerized systems for temperature control. They can maintain temperature within ±5°C, ensuring precise heat treatment.
Also, utilize robotic arms for handling metals between the furnace and quench bath, improving safety. With automation, the precision of your processes can be significantly improved, enhancing the overall product quality.
Advanced Equipment
Incorporating the latest tools in what is heat treatment process of metals is a must. High-tech furnaces that can reach 1,200°C offer better heat treatment. The use of induction heaters, which heat metals in less than 1 minute, could optimize your operations.
Similarly, top-quality quenching baths, able to cool metals rapidly without causing cracks, are invaluable. Remember, investment in cutting-edge technology can revolutionize your heat treatment processes, ensuring optimal results.
Lean Manufacturing
Lean manufacturing principles applied to heat treatment processes enhance efficiency. What is meant by heat treatment of metals is a process of heating and cooling metals.
This alters their physical properties, often improving hardness, ductility, and toughness. In a lean setup, the time spent on each process is minimized, saving time and reducing costs.
Quality Improvement
The process of heat treatment can drastically improve the quality of metal products. What is the purpose of heat treatment of metals is mainly to modify their characteristics.
For example, heat treatment can reduce metal brittleness and improve wear resistance. Quality enhancement results in fewer product failures and thus higher customer satisfaction.
Cycle Time Reduction
Heat treatment cycle time can be decreased by optimizing furnace efficiency. The right temperature and cooling time directly influence the final product properties.
What properties of metals can be improved after heat treatment include hardness, toughness, and ductility. Shorter cycle times translate to increased production rates and reduced costs.
Material Utilization
Effective use of materials is vital in heat treatment processes. What is the purpose of heat treatment of metal is to change its properties without altering the overall shape. By selecting appropriate temperatures and times for the heating and cooling processes, waste can be minimized, ensuring efficient material utilization.
Continuous Improvement
With continuous improvement, the heat treatment process can be constantly refined. Why heat treatment of metals is necessary is because it gives them desirable properties. By focusing on incremental changes, even a small reduction in heating or cooling time can result in significant productivity gains over time.
Predictive Maintenance
Heat treatment equipment, like furnaces, needs regular maintenance to ensure optimal performance. Regular checks and predictive maintenance can prevent unexpected failures, reducing downtime and ensuring uninterrupted production. With proper maintenance, the longevity and efficiency of the heat treatment equipment can be significantly enhanced.
Operational Excellence
An optimal heat treatment process focuses on the precise control of temperature and time. A range between 400°C to 1200°C is a common heat treatment window.
The timing varies depending on metal properties. Advanced thermocouples ensure accurate temperature measurement, enhancing the results. Furnace capacity, uniformity of heat distribution, and quick quenching contribute to high efficiency.
Cost Efficiency
Proper heat treatment can save significant costs in the long run. Ensuring correct alloy composition and reducing impurities minimize the need for rework. A well-maintained furnace reduces energy consumption, lowering operational costs.
A process like annealing, used for softening metals, can save on energy costs compared to other heat treatment methods. Implementing these strategies not only reduces costs but also improves the overall quality of the product.
Skill Training
Mastery in heat treatment processes demands specific skillsets. Training in understanding phase diagrams is critical. Understanding critical cooling rates for quenching different metals, such as the 4000°C per second for steel, is essential. Knowledge of tempering temperatures, typically around 150°C to 700°C, is vital. Training in advanced methods like cryogenic treatment, where metals are cooled down to -196°C, can give an edge.
Safety Enhancement
Safety is paramount in heat treatment operations. Furnaces operate at high temperatures, often exceeding 1000°C, posing a serious risk. Effective insulation, regular equipment checks, and strict adherence to safety protocols can significantly reduce hazards. Use of heat-resistant suits and face shields can protect personnel from severe burns.
Carbon Footprint Reduction
The heat treatment industry has a role in addressing climate change. By investing in energy-efficient equipment, energy consumption can be lowered, thus reducing CO2 emissions. Techniques like direct flame impingement, which directly heats the metal, cut down energy wastage. Vacuum heat treatment reduces the need for protective atmospheres, thereby reducing emissions.
Troubleshooting in Heat Treatment!

o Distortion Correction
Correcting distortion in the heat treatment of metals is critical. The process involves heating at 650°C, ensuring the metal’s uniform expansion. Then, you apply controlled cooling in stages. Tightly controlling temperature rates minimizes distortion.
To check the metal part’s straightness, a flatness gauge comes in handy. Achieving perfection in distortion correction is a testament to the expert handling of heat treatment processes.
o Cracking Prevention
Cracking is a common problem in heat treatment. To prevent it, there are careful considerations. The cooling rate, alloy composition, and hardening temperature must all be optimal. It’s about understanding the science of transformation temperatures.
For steel, it is critical to cool under the A1 temperature of 723°C. Consistency in applying these parameters is the key to crack prevention.
o Overheating Resolution
Avoiding overheating in heat treatment is crucial. Overheating changes grain size, leading to reduced toughness. Cooling at a regulated speed from a peak temperature, typically 850°C for steel prevents overheating. Experts must master temperature control to achieve the best outcomes. Ensuring such heat management reflects proficiency in the metal treatment industry.
o Decarburization Mitigation
Decarburization is a significant heat treatment issue. In high-temperature environments, steel loses carbon, affecting its hardness. An optimal furnace atmosphere helps to combat this.
Nitrogen-rich environments prevent carbon loss, and keeping temperatures less than 950°C reduces the decarburization rate. The correct furnace environment is vital in decarburization prevention.
o Hardness Issues
Hardness is an essential attribute in heat-treated metals. Quenching and tempering help to control it. Quenching involves rapid cooling from 900°C, and tempering requires reheating to fewer than 500°C. Controlling hardness values requires expertise and precision in the heat treatment process.
o Scaling Solution
Scaling is a surface defect in heat-treated metals. It’s caused by oxidation at high temperatures. To prevent it, you must maintain an optimal furnace atmosphere. Nitrogen or hydrogen atmospheres reduce scaling. Keeping the temperature below 900°C also helps. Efficient control of furnace conditions is essential for scaling prevention.
o Non-uniform Heating
Uniform heating is a must for heat treatment. Non-uniform heating results in varying hardness across the metal piece. Consistency in furnace temperature, typically around 900°C, is crucial. Skilled heat treatment professionals know the importance of uniform heating. Mastery in temperature control is a mark of excellence in this field.
o Material Defects
Material flaws pose a common challenge. For instance, inclusions – foreign bodies within the metal – can disrupt heat treatment. An expert eye spots these. Precise composition control prevents such issues, enhancing the output quality.
o Residual Stress
In the heat treatment process, the unequal expansion and contraction can cause residual stress. Alleviating this requires a careful control strategy. Stress relief operations can significantly improve the final product’s structural integrity.
o Quenching Problems
Quenching – rapid cooling – often results in warped or cracked metals. This arises from the abrupt temperature shift. Controlling the quenching speed helps mitigate these problems, ensuring a more consistent, defect-free output.
o Temperature Control
Proper heat management is pivotal in metal treatment. Metals must reach specific temperatures to change their properties. Precise, real-time temperature monitoring, with adjustments as necessary, is a must.
o Time Control
Just as crucial as temperature, treatment duration is key. Too short, properties don’t change. Too long, over-tempering occurs, weakening the metal. Experts calculate the optimum treatment time for each material.
o Atmosphere Management
Heat treatment often requires specific environmental conditions. Managing the gaseous atmosphere in the furnace is crucial. Using a carefully composed mix of gases can enhance the process, yielding a superior result.
o Equipment Issues
Equipment malfunction can disrupt heat treatment. Regular maintenance is essential. By using cutting-edge technology, like automated control systems, one can minimize such disruptions.
o Surface Contamination
Dirt, oil, or other contaminants can lead to an uneven heat distribution. Cleaning metals prior to heat treatment is crucial. A robust cleaning protocol can improve treatment results, promoting a smoother, more uniform finish.
Conclusion
Understanding the intricacies of heat treatment of metals opens doors to a world of improved metal properties. Knowledge of these principles empowers metal enthusiasts and professionals alike.
Remember, the process ensures a stronger, more durable future in various industries. To delve deeper into this intriguing subject, consider exploring the wealth of information available on KDMFAB. There, satisfy any curiosity that still sparks within.





Pingback: Decoding the Costs: How Much Does It Cost to Run an Infrared Heater?
Pingback: cialis lilly icos tadalafil
Pingback: buy custom essays
Pingback: write my social work essay
Pingback: i need help writing a compare and contrast essay
Pingback: essay writing service forum
Pingback: someone to write my essay
Pingback: help with argumentative essay
Pingback: essay writing service us
Pingback: best custom essay writing service
Pingback: top 10 essay writers
Pingback: custom essay service
Pingback: essays writing service
Pingback: help writing a compare and contrast essay
Pingback: cheap custom essays
Pingback: custom essay writing service org
Pingback: essays writing services
Pingback: viagra pharmacy 100mg
Pingback: phentermine india pharmacy
Pingback: va pharmacy
Pingback: online pharmacy no prescription needed
Pingback: rite aid 24 hour pharmacy store locator
Pingback: online cialis no prescription
Pingback: viagra for sale in india
Pingback: bph tadalafil
Pingback: cialis canada prices
Pingback: viagra generic europe
Pingback: cialis using paypal in australia
Pingback: viagra online prescription uk
Pingback: price of viagra per pill
Pingback: where can i get generic viagra online
Pingback: viagra 100mg online in india
Pingback: tadalafil combitic
Pingback: cialis cost per pill
Pingback: clopidogrel online pharmacy
Pingback: ralphs pharmacy
Pingback: cialis for bph insurance coverage
Pingback: viagra best price online
Pingback: over the counter cialis 2017
Pingback: buy female viagra online canada
Pingback: cheap prescription viagra online
Pingback: generic viagra for sale uk
Pingback: prices for viagra prescription
Pingback: viagra online buy usa
Pingback: cialis wikipedia
Pingback: on line cialis
Pingback: neurontin hematuria
Pingback: flagyl 375
Pingback: lyrica for pain
Pingback: furosemide veterinaria
Pingback: .75 mg semaglutide
Pingback: metformin and semaglutide
Pingback: prozac or zoloft
Pingback: gabapentin fiale
Pingback: how long does it take for escitalopram to kick in
Pingback: how long does it take for fluoxetine to get out of your system
Pingback: lexapro nursing implications
Pingback: can you take amoxicillin and doxycycline together
Pingback: order viagra pills
Pingback: azithromycin with tylenol